WHAT SHOULD I KNOW ABOUT FLUCELVAX® QUAD?
FLUCELVAX® QUAD is used in adults and children 6 months and older to prevent influenza, often called "the flu."
The National Advisory Committee on Immunization (NACI) encourages annual influenza vaccination for all Canadians who are able to have the vaccine.
Vaccination against influenza is recommended every year, for anyone wanting to lower their chance of catching influenza.
How does FLUCELVAX®
QUAD work?

FLUCELVAX® QUAD vaccine works by helping your body to protect itself against infection by the types of influenza viruses that are in the vaccine. The vaccine stimulates the body to make substances called antibodies.
Antibodies fight the influenza virus.
If you have been vaccinated, when you come into contact with the influenza viruses in the vaccine, your body is usually able quickly to destroy the virus, which may prevent you from getting influenza.
As with all vaccines, 100% protection cannot be guaranteed.
Learn More
- FLUCELVAX® QUAD is not made using eggs; therefore, there are no egg proteins in the vaccine.
- FLUCELVAX® QUAD is made using cell culture.
- The syringe and vial components do not contain latex. FLUCELVAX® QUAD is considered safe for use in persons with latex allergies.
- FLUCELVAX® QUAD pre-filled syringes contain no preservatives or antibiotics.
- FLUCELVAX® QUAD multi-dose vial formulation contains a preservative, but does not contain any antibiotics.

WHAT SHOULD YOU DISCUSS WITH YOUR DOCTOR?

DO NOT USE FLUCELVAX® QUAD if:
- Your child is under 6 months of age. FLUCELVAX® QUAD vaccine is only approved for use in children aged 6 months and older.
- You or your child have or have had an allergy to FLUCELVAX® QUAD or any of its ingredients.

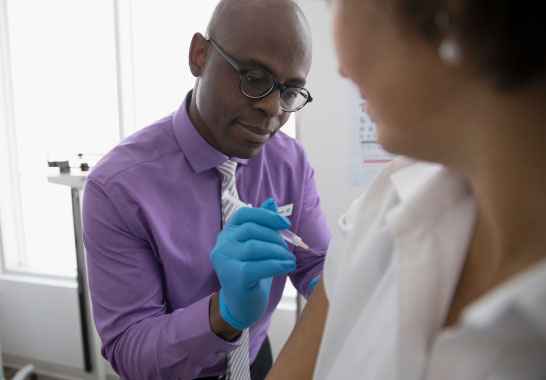
How do you take FLUCELVAX® QUAD?
HOW FLUCELVAX® QUAD IS GIVEN:
FLUCELVAX® QUAD is given as an injection into a muscle, usually in the upper arm.
USUAL DOSE:
FLUCELVAX® QUAD is given once every year as follows:
- Adults and children 9 years and over: one injection of 0.5 mL.
For children 6 months to less than 9 years old who are receiving influenza vaccine for the first time, it is recommended that a follow-up (booster) dose of FLUCELVAX® QUAD is given 4 weeks after the first dose.
If the follow-up dose is missed, talk to your healthcare professional and arrange another visit as soon as possible.
OVERDOSE
If you think you, your child, or a person you are caring for have been given too many doses of FLUCELVAX® QUAD or have been given it by mistake, contact your healthcare professional, hospital emergency department or regional poison control centre immediately, even if there are no symptoms.
What are some of the possible side effects from using FLUCELVAX® QUAD?
Tell your doctor if you or your child have side effects that bother you, including:
- Injection-site pain, reddening, hardening or swelling
- Headache
- Muscle or joint pain
- Tiredness
- Nausea, vomiting, diarrhea
- Loss of appetite
- Bruising
- Shivering
Younger children may also experience the following common or very common side effects:
- Injection-site tenderness
- Irritability
- Sleepiness
- Change of eating habits
- Fever
If you or your child experience either of these rare serious side effects, talk to your healthcare professional:
Anaphylaxis: Difficulty breathing, dizziness, a weak and rapid pulse, skin rash.
Allergic reaction: Rash, itching or hives on the skin, swelling of the face, lips, tongue, or other parts of the body.
REPORTING SUSPECTED SIDE EFFECTS
For the general public: Should you or your child experience a side effect following immunization, please report it to your doctor, nurse, or pharmacist.
Should you require information related to the management of the side effect, please contact your healthcare provider. The Public Health Agency of Canada, Health Canada and Seqirus cannot provide medical advice.

